The Nagoya Protocol: Access and Benefit Sharing

The Nagoya Protocol (NP) is an international agreement, part of the UN’s Convention on Biological Diversity (CBD), it came into force on the 12th October 2014. The NP recognises the rights of countries to regulate access and utilisation of their genetic resources and associated traditional knowledge through national legislation. Countries can negotiate the sharing of any benefits arising from the utilisation of these resources, these benefits can be monetary, or other e.g. training or collaboration. The NP aims to ensure that countries receive a “fair share” of any benefits and has the potential to positively influence biodiversity and its sustainable use.
Within the UK, the Competent Authority for Access and Benefit Sharing (ABS) is the Office for Product Safety and Standards (OPSS), who are appointed by Defra to advise, support and enforce the regulations.
Regulations: Nagoya Protocol on access and benefit sharing (ABS) – GOV.UK (www.gov.uk)
Generally, to be in scope of the Nagoya Protocol a genetic resource (in our case mainly algae and protozoa) must have been:
- accessed (collected) on or after October 12th 2014
- accessed (collected) from a country of origin that is a Party to the Nagoya Protocol, exercises sovereign rights (i.e. the high seas and Antarctica are out of scope), and has applicable access legislation in place
- utilised for Research and Development (whether commercial or non-commercial). Note that this covers a wide range of potential uses.
If a strain is in scope of the Nagoya Protocol, CCAP can only access it into our Collection if there is accompanying official documentation showing permission to collect the original sample, and clear permission to deposit the sample in a public Culture Collection, including specific details on any restrictions on distribution or permitted uses.
Potential depositors must exercise due diligence to ensure that any samples have been collected or obtained in accordance with any access and benefit sharing requirements of the country of origin, whether the depositor collected the original sample themselves or not.
Before Collecting Samples
To fulfil the obligations of the NP anyone wishing to collect samples of algae or protozoa must find out what international and national laws apply to sampling in the country from which samples are to be collected, including any sea areas that are part of the country’s territory. Note that even if a country is not a Party to the NP or has no access and benefit sharing measures in place under the NP, there may still be local or national laws that apply, for example in the UK samples can’t be collected from Sites of Special Scientific Interest without prior consent.
To find out if a country is a party to the NP and for further information on any regulations there is a clearing-house website, the ABSCH:
The ABSCH contains information on any access and benefit sharing (ABS) regulations or laws a country has in place. Note, however, that an absence of regulations listed on the ABSCH isn’t conclusive – countries are largely responsible for updating their own records on the website, and it is always recommended to check directly with the country as part of your due diligence. Each country has one or two national contacts, the National Focal Point (NFP) and/or Competent National Authority (CNA), and the ABSCH has details of email addresses and often web links for further information. When making contact, explain what you wish to collect and from where, and give details of what you intend to do with the samples, you should be given advice on what permits and other documents are needed, if any.
After discussion with the relevant national authority, two documents may be supplied:
- PIC (Prior Informed Consent): this is a permit for accessing the genetic resource.
- MAT (Mutually Agreed Terms): these are negotiated with the CNA, or a community, in the country of origin, prior to the access/collection of any material. The terms may include permitted uses of the samples, and any benefit sharing, e.g. an agreement to share any scientific papers and data with the origin country, training/education programmes, or a share of any profits should research on a sample lead to a commercial product.
When the PIC and MAT information is published to the ABSCH, this may be in the form of an IRCC, or Internationally Recognised Certificate of Compliance.
These documents prove that the material was legally accessed and will also determine permitted uses and terms of benefit sharing, or confirmation that there are no restrictions. Copies of the documents must be transferred along with the material to any subsequent users and must be retained by each user for at least 20 years after the end of the period of use.
Depositing strains with CCAP
If you wish to deposit a strain in scope of the NP with CCAP, but there is no mention in the documents of depositing samples in a public Collection, then please contact the relevant national authority and often the MAT can be renegotiated. In order to accept a strain for standard deposit we need clear permission for the sample(s) to be deposited either specifically in CCAP, or in any Culture Collection in any country; and clear permission to distribute samples (and any restrictions, for example distribution for non-commercial use only).
If a strain is in scope of the NP but has no documentation, it may be possible to retrospectively obtain permission and a MAT by contacting the relevant national authority prior to deposit.
CCAP will also require copies of any documents including PIC and MAT (or IRCC), and will send these to anyone to whom we supply samples of the deposit.
UK Genetic Resources
Although a party to the NP, the UK has chosen not to exercise sovereign rights for accessing its genetic resources under the NP. There is no requirement to obtain a PIC or MAT for any utilisation of UK genetic resources, however depending on the collection site there may be other UK legislation which should be taken into account prior to any sampling.
If a strain comes under the scope of provider country legislation under the NP there will be information in the strain data in our online catalogue.
Our aim is to have a clear indicator of NP status for every strain in the CCAP catalogue, however in many cases this means going through original accession sheets and sometimes additional detective work to find dates, we currently have an indicator for about 45% of strains.
Strains in scope of the NP
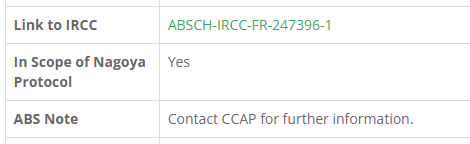
Strains that are in scope of the NP are noted as such on our web catalogue and cannot be purchased online. If there is any documentation available online it will be linked, see example below from strain CCAP 1361/11 Bangia sp.
In this case the information in the IRCC on utilisation of the sample is brief and there is no explicit permission for distribution of samples. CCAP can store the strain in our collection as storage is not research and development and is therefore not in scope of the NP ABS regulations. If a user wished to acquire this strain it would be necessary for them to contact the relevant authority (in this case France) and enquire about renegotiating terms, before accessing the strain.
Any future accessions of strains in scope of the NP must have documentation giving clear permission for the strain to be held in CCAP and to be distributed, and with details of any restrictions.
Copies of any associated documents and permissions will be available and will be transferred with the strain if purchased. These will outline how the strain can be utilised under the terms of the MAT/provider country legislation, and any information on benefit sharing.
These documents must be stored for at least 20 years after the end of the period of use. If the strain is passed on to a third party (assuming this is allowable under the MAT) copies of the documents must accompany the strain.
The user can contact the Competent National Authority (CNA) or National Focal Point (NFP) of the country of origin if any clarification or amendments are required. Details of the contacts for each country can be found on the ABS Clearing-House website:
Strains that are not in scope of the NP
If a strain that is out of scope of the NP has any ABS restrictions that CCAP is aware of, this will be noted and this information will be passed to the user. In many cases this information will not have been supplied to the CCAP and it is the responsibility of the user to ensure the commercial use of any strains or resultant product(s) do not infringe national laws or regulations.
From our Terms and Conditions of Culture Supply:
If the recipient desires to use the Material (or modifications) for defined commercial purpose(s), it is incumbent upon the recipient, in advance of such use and providing that the Country of Origin is a signatory to the CBD, to negotiate in good faith with the depositor or appropriate authority in the Country of Origin, the terms of any benefit sharing in compliance with the CBD.
Further information and guidance:
- The CBD: The Nagoya Protocol
- ABS Clearing House
- GOV.UK Regulations: Nagoya Protocol on access and benefit sharing (ABS)
- Defra Guidance on the UK Access and Benefit Sharing Regulations – published 2022
- EMBRC Guide to ABS Compliance: Recommendations To Marine Biological Resources, Collections’ And Users’ Institutions
- MIRRI (Microbial Resource Research Infrastructure) ABS Best Practice Manual
Page last updated 19/01/2024