References [ 22 ]
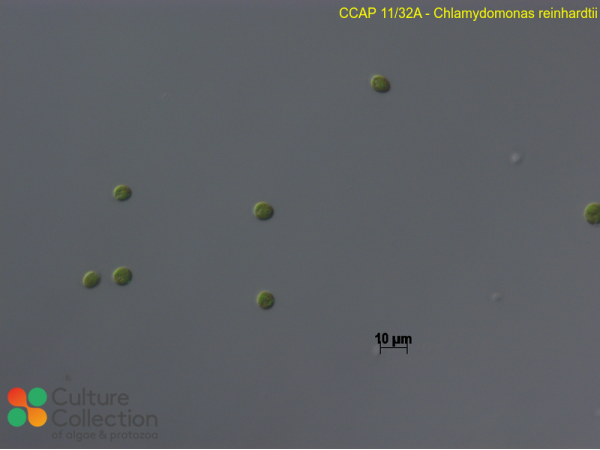
Pröschold T, Harris EH & Coleman AW (2005) Portrait of a species: Chlamydomonas reinhardtii Genetics 170: 1601-1610.
Hellio C, Veron B & Le Gal Y (2004) Amino acid utilization by Chlamydomonas reinhardtii: Specific study of histidine. Plant Physiology and Biochemistry 42(3): 257-264.
Dean AP, Nicholson JM & Sigee DC (2008) Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: An FTIR microspectroscopy study. European Journal of Phycology 43(4): 345-354.
Cullimore JV & Sims AP (1980) An association between photorespiration and protein catabolism: Studies with Chlamydomonas. Planta 150: 392-396.
Dean AP, Sigee DC, Estrada B & Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresource Technology 101: 4499-4507.
Carslake D, Townley S & Hodgson DJ (2009) Predicting the impact of stage-specific harvesting on population dynamics. Journal of Animal Ecology 78: 1076-1085.
Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès, Li-Beisson Y & Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterisation, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnology 11: 7.
DOI: none
Turmel M, Cote V, Otis C, Mercier J, Gray MW, Lonergan KM & Lemieux C (1995) Evolutionary transfer of ORF-containing group I introns between different subcellular compartments (chloroplast and mitochondrion). Molecular Biology and Evolution 12: 533-545.
DOI: none
Birungi ZS & Chirwa EMN (2014) The kinetics of uptake and recovery of lanthanum using freshwater algae as biosorbents: Comparative analysis. Bioresource Technology 160: 43-51.
Esperanza M, Cid A, Herrero C & Rioboo C (2015) Acute effects of a prooxidant herbicide on the microalga Chlamydomonas reinhardtii: Screening cytotoxicity and genotoxicity endpoints. Aquatic Toxicology 165: 210-221.
Esperanza M, Seoane M, Rioboo C, Herrero C & Cid A (2015) Chlamydomonas reinhardtii cells adjust the metabolism to maintain viability in response to atrazine stress. Aquatic Toxicology 165: 64-72.
Fernéndez-Naveira A, Rioboo C, Cid A & Herrero C (2016) Atrazine induced changes in elemental and biochemical composition and nitrate reductase activity in Chlamydomonas reinhardtii. European Journal of Phycology 51: 338-345.
Esperanza M, Seoane M, Rioboo C, Herrero C & Cid A (2016) Early alterations on photosynthesis-related parameters in Chlamydomonas reinhardtii cells exposed to atrazine: A multiple approach study. Science of the Total Environment 554-555: 237-245.
Lachmann SC, Maberly SC & Spijkerman E (2016) Ecophysiology matters: Linking inorganic carbon acquisition to ecological preference in four species of microalgae (Chlorophyceae). Journal of Phycology 52: 1051-1063.
Martín-Betancor K, Aguado S, Rodea-Palomares I, Tamayo-Belda M, Leganés F, Rosal R & Fernández-Pinas F (2017) Co, Zn and Ag-MOFs evaluation as biocidal materials towards photosynthetic organisms. Science of the Total Environment 595: 547-555.
González-Pleiter M, Rioboo C, Reguera M, Abreu I, Leganés F, Cid A & Fernández-Pinas F (2017) Calcium mediates the cellular response of Chlamydomonas reinhardtii to the emerging aquatic pollutant Triclosan. Aquatic Toxicology 186: 50-66.
Esperanza M, Houde M, Seoane M, Cid A & Rioboo C (2017) Does a short-term exposure to atrazine provoke cellular senescence in Chlamydomonas reinhardtii? Aquatic Toxicology 189: 184-193.
González-Pleiter M, Rioboo C, Reguera M, Abreu I, Leganés F, Cid A & Fernández-Pinas F (2017) Calcium mediates the cellular response of Chlamydomonas reinhardtii to the emerging aquatic pollutant triclosan. Aquatic Toxicology 186: 50-66.
Pröschold T, Darienko T, Krienitz L & Coleman A (2018) Chlamydomonas schloesseri sp. nov. (Chlamydophyceae, Chlorophyta) revealed by morphology, autolysin cross experiments, and multiple gene analyses Phytotaxa 362: 21-38.
Tamil Selvan S, Govindasamy B, Muthusamy S & Ramamurthy D (2019) Exploration of green integrated approach for effluent treatment through mass culture and biofuel production from unicellular alga, Acutodesmus obliquus RDS01 International Journal of Phytoremediation -: -.
Esperanza M, Seoane M, Rioboo C, Herrero C & Cid A (2019) Differential toxicity of the UV-filters BP-3 and BP-4 in Chlamydomonas reinhardtii: A flow cytometric approach Science of the Total Environment 669: 412-420.
Singh T, Sehgal A, Singh R, Sharma S, Bahadur Pal D, Tashkandi HM, Raddadi R, Harakeh S, Haque S, Srivastava M, Hassan AA, Srivastava N & Gupta VK (2023) Algal biohydrogen production: Impact of biodiversity and nanomaterials induction Renewable and Sustainable Energy Reviews 183: 113389.